What does mole number mean?
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is “mol”.
Avogadro's number is the number of units in one mole of a substance, or 6.02214076 × 1023. This number is also called the Avogadro constant.
To correctly estimate the number of moles, n , of a substance of a specific mass, m , (in grams), you need to follow the grams to moles formula: n = m / M , where: M is the molar mass of this material.
- First you must calculate the number of moles in this solution, by rearranging the equation. No. Moles (mol) = Molarity (M) x Volume (L) = 0.5 x 2. = 1 mol.
- For NaCl, the molar mass is 58.44 g/mol. Now we can use the rearranged equation. Mass (g) = No. Moles (mol) x Molar Mass (g/mol) = 1 x 58.44. = 58.44 g.
One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro's number or Avogadro's constant. The concept of the mole can be used to convert between mass and number of particles..
A mole is defined as that amount of substance which contains Avogadro's number (6.022140857×1023) number of atoms if the substance is atomic or Avogadro's number of molecules if the substance is molecular. For example, 6.022140857×1023 molecules of H2O will form 1 mole of H2O.
The Mole in Elements, Molecules, and Compounds
We already know that one mole of an element contains 6.02214 x 1023 atoms (Avogadro's number), so two moles are the same as 2 * 6.02214 x 1023 atoms = 12.04428 x 1023 carbon atoms.
The amount of substance is given as the number of moles in the sample. For most practical purposes, the numerical value of the molar mass expressed with the unit gram per mole is the same as that of the mean mass of one molecule of the substance expressed with the unit dalton.
A mole is a unit of measurement in chemistry. Here is the official definition: One mole of something (say, atoms, or raindrops) is equal to as many of that something as there are atoms in 12 grams of the isotope carbon-12.
...
Solution.
| Known Information | Multiply By | Result |
|---|---|---|
| Moles of substance (mol) | Avogadro's constant (atoms/mol) | Atoms (or molecules) |
| Mass of substance (g) | 1/Molar mass (mol/g) × Avogadro's constant (atoms/mol)) | Atoms (or molecules) |
How do you make 1 mole?
A mole consists of 6.02×1023 molecules or atoms. Molecular weight (MW) is the weight of one mole of a chemical. Determine MW using a periodic table by adding the atomic mass of each atom in the chemical formula.
You can think of “a mole” the same way you think of “a dozen.” You're probably familiar with a dozen eggs, or chickens or planets. Moles are no different. You can have a mole of molecules or people or cheeseburgers. But there are a lot more than twelve things in a mole — there are 6.02 x 1023.
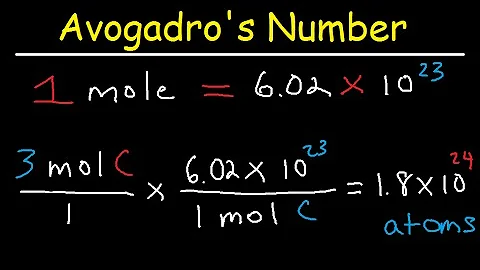
On average, moles grow to 4.4 to 6.25 inches (11.3 to 15.9 centimeters) long from snout to rump. Their tails add 1 to 1.6 inches (2.5 to 4 cm) of length. They typically weigh 2.5 to 4.5 ounces (72 to 128 grams), according to the Mammal Society (opens in new tab). The American species is a little on the larger side.
The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. 12.00 g C-12 = 1 mol C-12 atoms = 6.022 × 1023 atoms • The number of particles in 1 mole is called Avogadro's Number (6.0221421 x 1023).
...
= 3 x 28.01 = 84.03 grams.
| FORMULAS Related Links | |
|---|---|
| Integral Calculus Formulas List | Orthocentre Of A Triangle Formula |
An exact number! Infinitely precise. That many elementary particles, be they atoms, molecules, ions, photons, etc., is known as a mole of that substance or entity. IUPAC's wording: One mole contains exactly 6.02214076 × 1023 elementary entities.
6.02×10^23 is called the Avogadro Constant and it defines the number of particles (atoms or molecules) in one mole of substance. In simple terms, a mole of anything is always 6.02×10²³.
The number of moles can be given by the unit “mol”. The term mole can be used with any chemical species such as atoms, molecules, ions, etc. Therefore, one mole of sulfur means 1 mol of sulfur atoms. One mole of carbon dioxide means, 1 mol of CO2 molecules.
The mole, symbol mol, is the SI unit of amount of substance. One mole contains exactly 6.022 140 76 x 1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol–1 and is called the Avogadro number.
It's important to get a new or existing mole checked out if it: changes shape or looks uneven. changes colour, gets darker or has more than 2 colours. starts itching, crusting, flaking or bleeding.
Why is a mole 6.02 x10 23?
The MOLE (mol) is a unit of measurement that is the amount of a pure substance containing the same number of chemical units (atoms, molecules etc.) as there are atoms in exactly 12 grams of carbon-12 (i.e., 6.022 X 1023).
The mole is an amount unit similar to familiar units like pair, dozen, gross, etc. It provides a specific measure of the number of atoms or molecules in a bulk sample of matter. A mole is defined as the amount of substance containing the same number of discrete entities (atoms, molecules, ions, etc.)
1) Because atoms and molecules are so small, the mole concept allows us to count atoms and molecules by weighing macroscopically small amounts of matter. 2)It establishes a standard for determining the stoichiometry of reactions. 3)It provides an explanation of the properties of gases.
Although common moles are pretty normal and shouldn't always be a cause for concern, having more than 50 common moles on your body puts you at a drastically increased risk of skin cancer. If you have a lot of moles on your body, regardless of the type of moles that they are, you should consult your physician.
Redness or a new swelling beyond the border of the mole. Change in sensation, such as itchiness, tenderness, or pain. Change in the surface of a mole – scaliness, oozing, bleeding, or the appearance of a lump or bump.
Border that is irregular: The edges are often ragged, notched, or blurred in outline. The pigment may spread into the surrounding skin. Color that is uneven: Shades of black, brown, and tan may be present. Areas of white, gray, red, pink, or blue may also be seen.
A mole, abbreviated as mol, is a measurement of amount used by scientists. One mole is equal to 6.022x1023 units. A mole is an important unit because on the periodic table a mole of a substance is equal to its atomic mass in grams.
WHAT'S A MOLE? Avogadro's Number is the number of atoms, molecules, or other objects that makes up one mole of a substance. For example: 6.022 x 1023 hydrogen atoms represent one mole of hydrogen.
Formula of Avogadro's Number
The numbers of atoms required are such that the number of grams of a substance turns out to be equal to the substance's atomic mass. NA = 6.0220 x 1023 mol-1. The word mole refers to the Avogadro's number of a substance. For example, a mole of carbon-12 atoms happens to be 12 grams.
Even if they are tiny (like a freckle) you should count it.
How many moles is normal?
A common mole is a growth on the skin that develops when pigment cells (melanocytes) grow in clusters. Most adults have between 10 and 40 common moles. These growths are usually found above the waist on areas exposed to the sun.
Moles have an extra thumb on each forelimb that helps them dig. Moles capture earthworms and store them for later—sometimes hundreds at a time. With fleshy, finger-like protrusions on its face, the star-nosed mole can catch and eat prey in just 230 milliseconds—the fastest of any mammal.
Why is the mole unit so important? It represents the link between the microscopic and the macroscopic, especially in terms of mass. A mole of a substance has the same mass in grams as one unit (atom or molecules) has in atomic mass units.
Avogadro's law states that, at a constant temperature and pressure, the volume and number of moles of a gas are directly proportional. This means that if the number of moles increases, the volume of the gas must also increase at a constant rate. To remember this relationship, we can think about blowing up a balloon.